The Basics
- John dalton was an English scientist that studied atoms and gases.

- He discovered the gas laws.
- These laws led to the first atomic theory.
- Dalton communicated his theory to J.J. Thompson.
- Some of Dalton's theories are still believed today.
- Dalton's work was not believed initially and it took over half a century for opposition to die off.
- His work set the stage for continued investigation into atoms.
Dalton's Atomic Theory: 6 Postulates
- Atoms can't be broken down.
- All atoms of a given element are the same.
- Atoms of different elements have different properties, including mass.
- Atoms of an element are not destroyed or changed in chemical reactions. This is known as the "Law of Conservation of Energy!"
- Compounds form when atoms of more than one element combine.
- In a compound, the relative number and kind of atoms are constant. This is known as the "Law of Definite Proportions."
6 Postulates: True or False
1. Atoms can't be broken down.
False, today we know that atoms can be broken down into smaller particles such as protons, neutrons, and electrons. Also we can split atoms and create nuclear bombs!
2. All atoms of a given element are the same.
False, some atoms in some elements have different masses.
3. Atoms of different elements have different properties, including mass.
True! This is still believed today.
4. Atoms of an element are not destroyed or changed in chemical reactions.
True!
5. Compounds form when atoms of more than one element combine.
True!
6. In a compound, the relative number and kind of atoms are constant.
True!
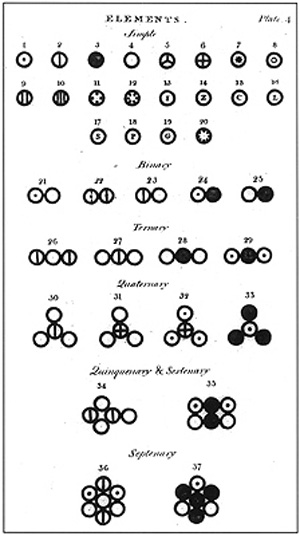
Dalton's Atom
Dalton proposed that everything was made up of small indivisible particles called atoms. Even though the idea of atoms has been studied in the past he was one of the first to create 6 postulates to define the atom. He believed that if you cut something in half enough, you would eventually get something too small to cut anymore. He suggested that these small particles, atoms, made up all matter.
Dalton's atomic model looked a lot like this:
Video!







