- J.J. Thompson was an English physicist.
- Thomson was awarded the 1906 Nobel Prize in Physics for the discovery
 of the electron and for his work on the conduction of electricity in gases.
of the electron and for his work on the conduction of electricity in gases. - Although many scientists believed that atoms were built from smaller particles that where the size of the smallest atom, hydrogen, thompson was the first to propose that these particles where over 1,000 times smaller than an atom.
- Thompson conducted the famous Cathode Ray Tube Experiment which led to the discovery of the electron.
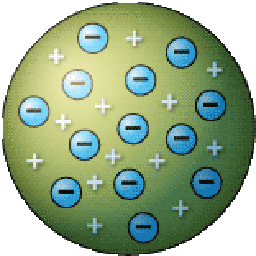
- Thompson created the "Plumb Pudding Model" of the atom. This displayed the electrons stuck inside the atom, like plum pudding.
- Thompson's work on the atom led to many more discoveries that influenced work today.
- Thompson's pupil, Rutherford, would continue working with the atom and make more important developments to how we see atoms today.
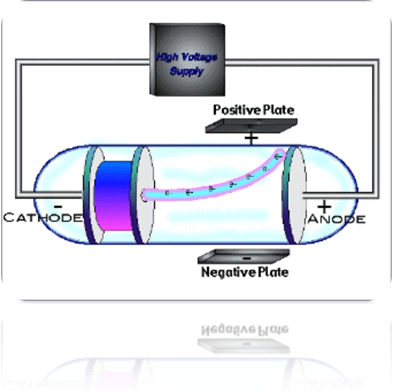
Thompson preformed an experiment studying electrical discharge through a tube called the cathode ray tube. This tube can be found in any older TV set or computer. If you put a magnet to the glass of the tube, the stream of electricity is disrupted. This is why when you put a magnet up to a TV it messes up the picture.
The cathode ray tube has most of the air sucked out. It produces a stream of light that moves from one metal disk on one side to another metal disk on the other. Thompson put a negatively charged plate on one side and a positively charged plate on the other. The ray was disrupted and moved away from the negative plate and towards the positive. This meant that the stream was made of negatively charged particles! He eventually postulated that these particles were thousands of times smaller than the already tiny protons. From this experiment he came up with the next atomic model: the plum pudding model.
Thompson visualized that these newly discovered electrons were stuck inside the atom. He called this the Plumb pudding model. he said that the electrons where placed inside the atom, just like plumb pudding (a British food) and were in no particular order.
His model looked like this: